Characterization of mesenchymal stem cells
The potential clinical use of mesenchymal stem cells (MSC) in equine veterinary medicine has been increasingly explored the past few years. Initially, little or no standardization for the isolation and characterization of equine MSC was present, in marked contrast to the detailed guidelines described for the characterization of human MSC. First, the isolation protocol for umbilical cord blood-derived MSC was optimized by comparing four isolation methods (hydroxyethyl starch, ammonium chloride, Ficoll-Paque and Percoll), and culture conditions such as cell density, culture medium and serum coating of the wells (Figure 1).
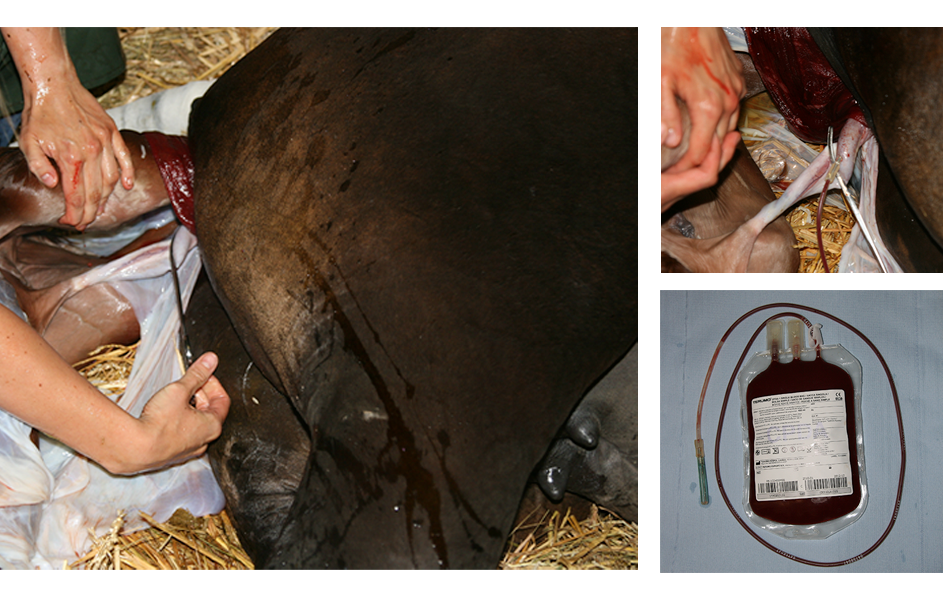
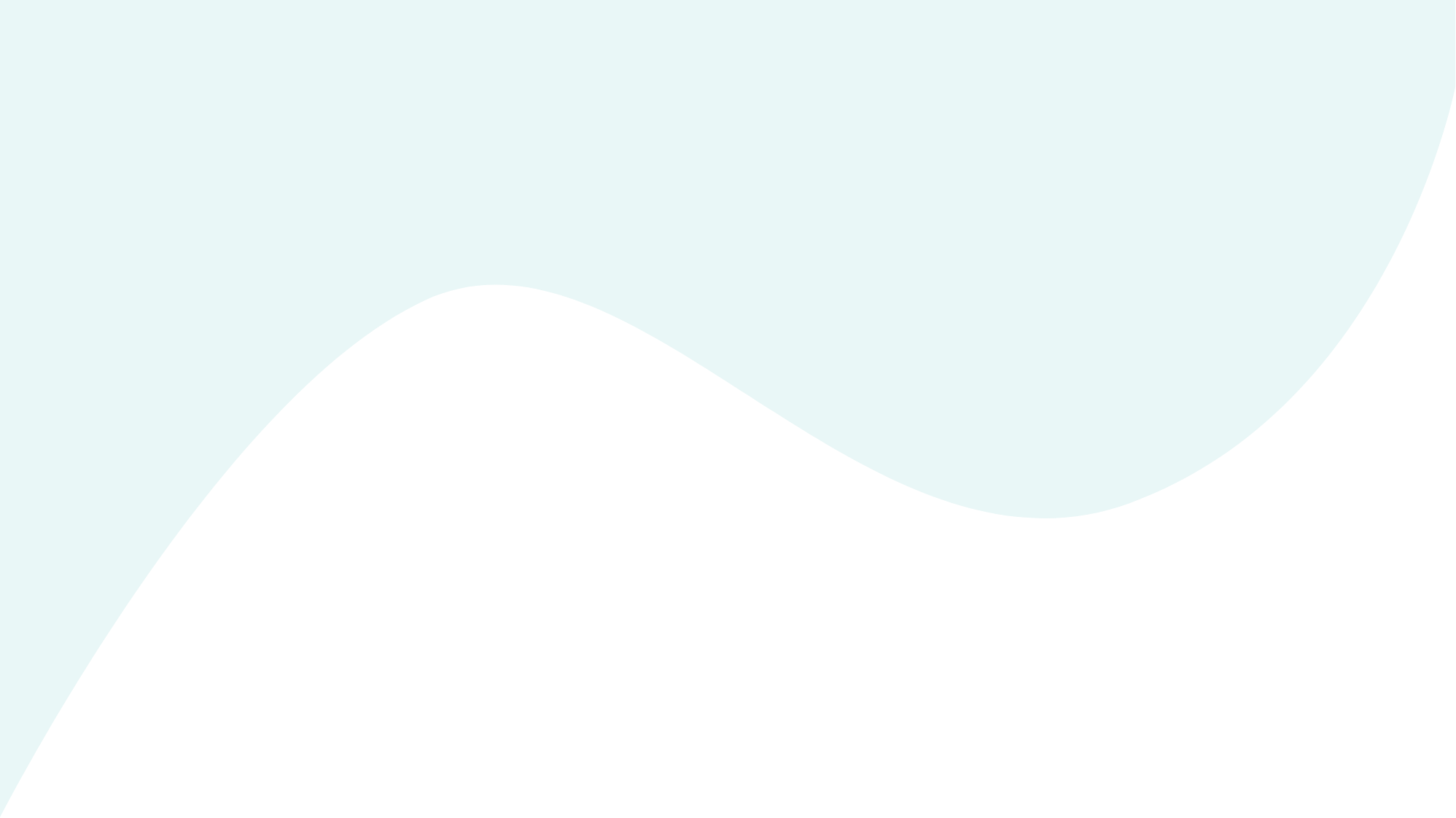
Figure 1. Umbilical cord blood is a non-invasive source for MSC isolation that can be collected easily at parturition before the umbilical cord ruptures.
To confirm the mesenchymal identity, isolated MSC were differentiated towards the osteogenic, chondrogenic and adipogenic lineage (Figure 2), and immunophenotyped by multicolor flow cytometry based on their expression of different cell protein markers.
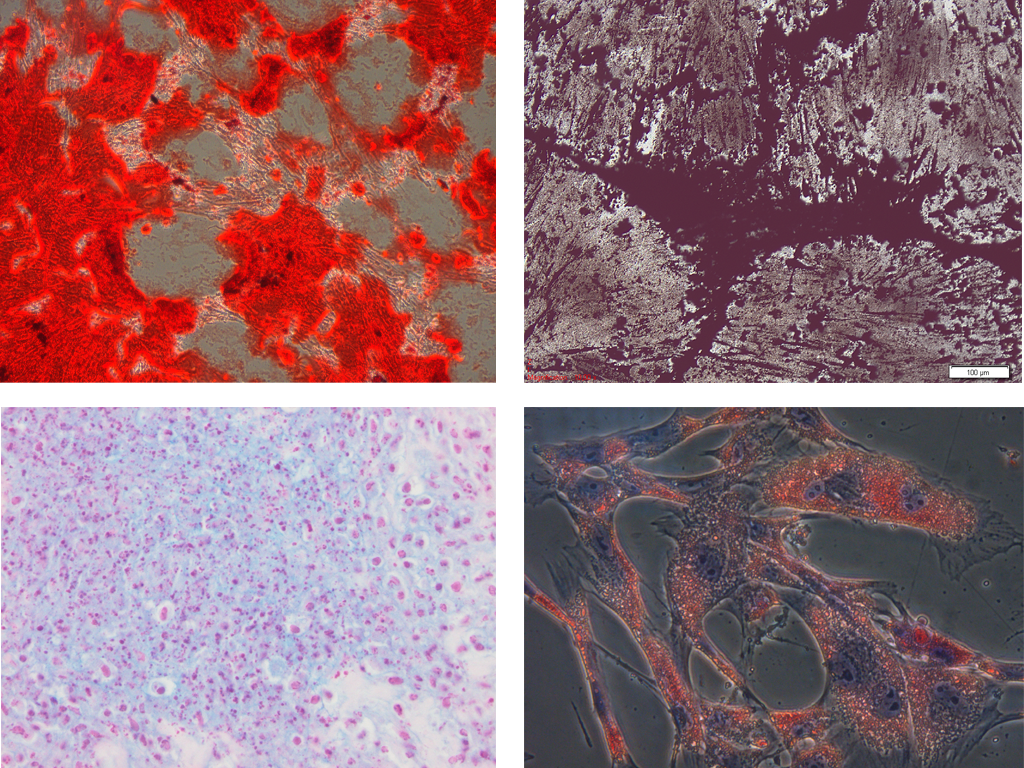
Figure 2. Multi-lineage differentiation potential of equine mesenchymal stem cells: (1) osteogenic differentiation as confirmed by the Alizarine Red staining and (2) the Von Kossa staining, (3) chondrogenic differentiation as confirmed by the Alcian Blue staining and (4) adipogenic differentiation as confirmed by the Oil Red O staining (De Schauwer et al., 2011).
Since MSC markers share many common features with endothelial, epithelial and muscle cells, a panel of antigens is necessary to unequivocally identify MSC. As such, human MSC must express CD29, CD44, CD73, CD90 and CD105 and lack expression of CD14, CD34, CD45, CD79α and MHC II. The limited availability of monoclonal antibodies for immunophenotyping cells of large and small animal species is a major factor complicating the immunophenotypic characterization of MSC in veterinary medicine. Thirty commercially available monoclonal antibodies were validated for recognizing equine epitopes using equine mononuclear cells, equine lymphocytes or equine endothelial cells as appropriate positive control cells (Figure 3). Subsequently, a multicolor flow cytometric protocol to immunophenotype equine MSC was developed to simultaneously demonstrate the co-expression of specific MSC markers and the absence of hematopoietic antigen expression.
Figure 3. Flow cytometric and confocal fluorescence microscopic analyses of the cross-reacting monoclonal antibodies based on the appropriate equine positive control cells (De Schauwer et al., 2012).
In conclusion, we were the first to properly immunophenotype equine MSC.
Moreover, we optimized derivation of MSC from other sources such as bone marrow, adipose tissue, peripheral blood and Wharton’s jelly, and in other species, including bovine and canine.